Alexandra
TPF Noob!
*sigh*... needed a break from the physics homework.
(oh, and if anyone knows how these split into ions, all ideas welcome)
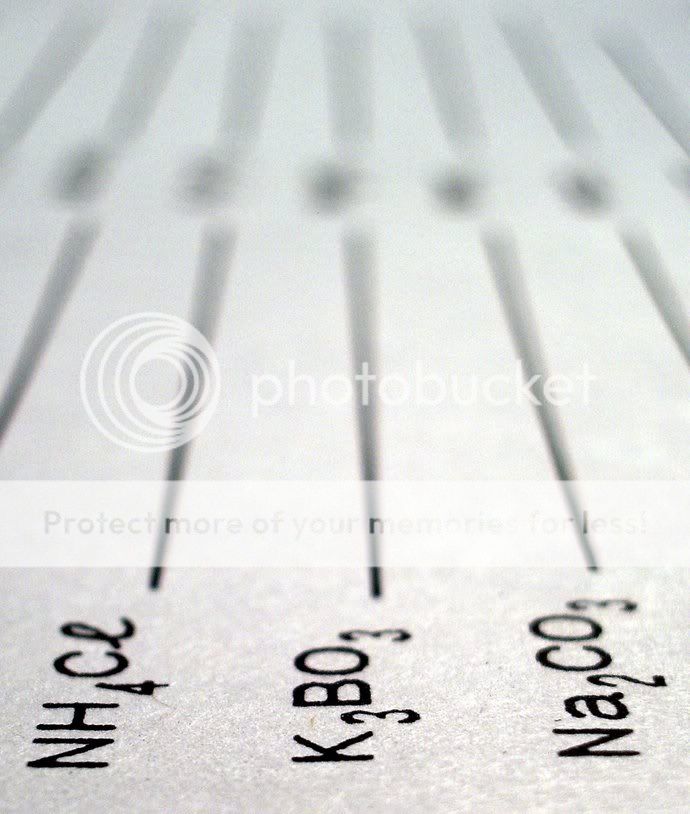
(oh, and if anyone knows how these split into ions, all ideas welcome)

Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature currently requires accessing the site using the built-in Safari browser.


Alexandra said:and if anyone knows how these split into ions, all ideas welcome)
 .
.Alexandra said:Rob, Brit, Jon, thanks a whole lot for putting some fun into my evening
Brit, I tried it horizontal, but honnestly, i don't really like it. It looks like the sheet is standing in front of me like some kinda wall or something... and it looks intimidating (or maybe i'm really too concerned about cause i don't get it, lol). Though, I have a version of it which is absolutely out of focus... really reflects the way i feel about it.
...oh, and slightly going off topic: for k3bo3: i suppose the 3k+ will split out from 3bo- which will become a radical. am i right?